Rare earth metals are found in almost every modern product. The Toyota PRIUS contains about thirty pounds of them and a typical wind turbine contains as much as 500 pounds. China sets the price and China is our only source. If you are concerned about Green Energy and/or American National Defense, this should worry you.
Often abbreviated as just “rare earths,” these metals today are found in the world’s strongest permanent magnets, electric motors, loudspeakers, computers, hard disc drives, smartphones, lasers, batteries, camera lenses, catalysts and catalytic converters, wind turbines, smart bombs, drones, guided missiles, the best red, green, and blue phosphors for color TV screens, fiber optics, dental lasers, medical radiation therapy, military infrared lasers, naval sonar, aircraft and aerospace, spark plugs, gas mantles, metal alloys, PET scan detectors, light and fluorescent bulbs, LEDs, microwave filters, MRI contrast agents, strain gauges, welding safety goggles, and many more applications.
There are seventeen rare earths, most with funny-sounding names (we’ll get to that). They are so similar chemically that it took centuries to separate and identify them and give them names. America during WWII was the first country to purify them on an industrial scale, to characterize them, and to discover their chemical and physical properties and potential uses. America held the monopoly until the 1990’s when a series of blunders by companies like General Motors, thinking short-term profits, with complicity from the Clinton Administration let the technology slip away to the Chinese, the latter always thinking long term.
By lowering the price of the rare earths below market value for many years, China killed all competition. At this point, China has the industrial might and most of the world’s reserves of rare earth ores. It would cost many billions of dollars and take decades for us to regain a competitive position. From now on we would be well-advised to stay on friendly terms with China lest they pull a boycott of the rare earth metals.
Allow me to introduce the these extraordinary metals, and then I will tell you about my adventures in the summer of 1958, working as a technician producing some of them in the Atomic Energy Commission (AEC) Ames Laboratory in Ames, Iowa.
In the Table I have listed 14 of the 15 rare earths, going from Atomic Number (number of protons in the nucleus) 57 through 71. (Number 61, called Promethium, is omitted because it is radioactive and doesn’t exist in nature.) Two additional elements, Numbers 21 and 39 are normally thrown in, Scandium and Yttrium, making a total of 16, simply because they are so similar chemically to the rare earths and are found together with them in nature.
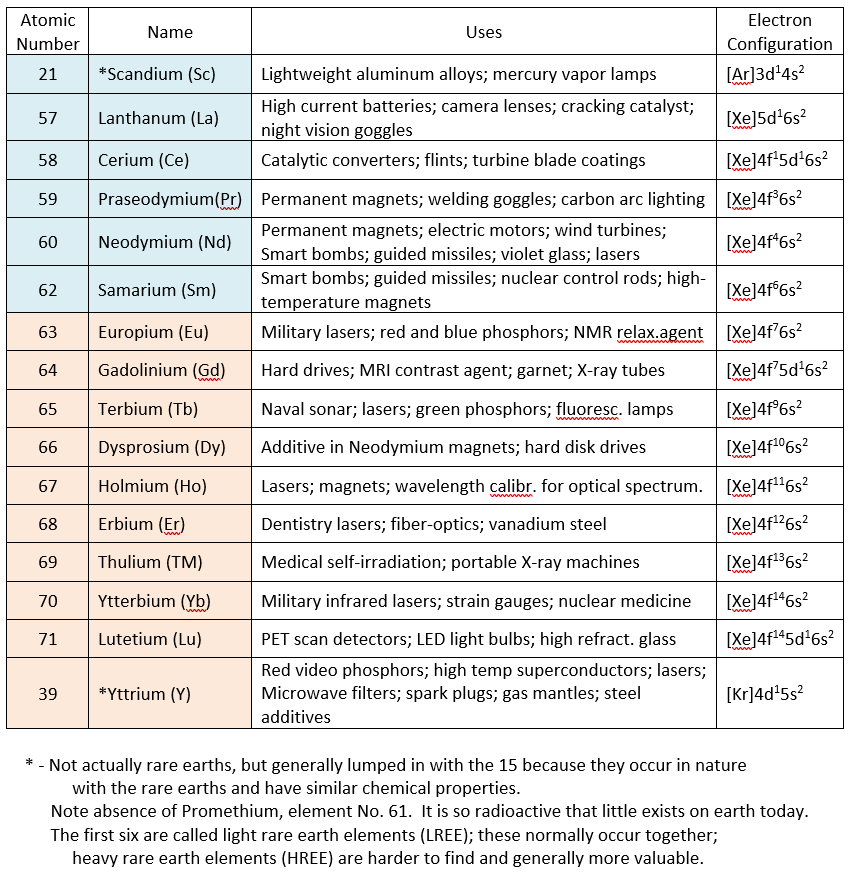
There is a song by Tom Lehrer in which he recites the names of these elements rapid-fire, so maybe we could all learn them too. Scandium, Lanthanum, Cerium, Praseodymium, Neodymium, Samarium, Europium, Gadolinium, Terbium, Dysprosium, Holmium, Erbium, Thulium, Ytterbium, Lutetium, and Yttrium. Much of the early work was done in the late 1700s by the Swede Arrhenius and the Finn Gadolin using an ore called “ytterbite” from a quarry in Ytterby, Sweden. Thus you can see how many of the odd elemental names arose from this brilliant “odd couple.” As separations improved the number of rare earth names increased. For example, a supposed element known as “didymium,” used for decades in glassblowers’ goggles, was eventually separated into “praseodymium” (green didymium) and “neodymium” (new didymium).
The Table also lists some of the best-known uses of each rare earth metal. Don’t be too confused by the electronic configurations of these elements also listed in the Table. It just gives the number of electrons in the various orbitals (from s, p, d, and f) of all elements’ outer shells, sometimes on top of completed rare gas shells (He, Ne, Ar, Kr, Xe, Rn, and Uuo). The main point is that Elements 57 through 71 differ mainly in the number of electrons in their outermost f orbitals. That produces only the most minute differences in chemical properties as we’ve already pointed out, and provides the theoretical explanation for rare earth chemistry.
The Atomic Energy Commission (AEC) Ames Laboratory, Ames, Iowa.

Ames Laboratory, Ames, Iowa (Google Street)
On the campus of bucolic Iowa State University there is a complex of buildings which during WWII started to work on various aspects of the Manhattan Project for the AEC. With Prof. Frank H. Spedding as Director, the first project in 1942 was the production of tons of high purity uranium ingots to be used for the first successful atomic pile by Enrico Fermi under the stands of the University of Chicago, Illinois football stadium. By 1945 the Ames Lab had shipped 1000 tons of uranium ingots for isotopic enrichment at Oak Ridge, Tennessee, and Hanford, Washington.
The separation of the rare earths by ion-exchange became the top specialty of the Ames Lab. Using the same principle as most home water softeners, ion-exchange resins polymerized into uniform spherical beads covered with positively charged ions are packed into columns. A chromatographic column is a tube, usually made of glass, with a filter in the bottom and a way to control the flow rate. The mixture of compounds to be separated is deposited into a band on the top of the column, then washed with various solutions called “eluting agents”. A competition is established in which the cations are torn between sticking to the column and being induced to accompany the eluting agents down the column. After thousands of competition cycles, the sample begins to separate into as many as seventeen bands which can be collected in separate vessels. After extensive testing of various types of column packings, different column heights and diameters, different temperatures, and scores of eluting agents, several landmark papers were published in the late 1940’s describing the process for purifying rare earths on a gram scale.

2709 Lincoln Way, Ames, Iowa (Google Street)
In the summer of 1958, I was one of several Carleton chemistry majors who, thanks to our excellent Placement Service were given an opportunity to work in the Ames Lab. This included my good friend and classmate Jim Miller. Four of us were given a corner apartment to rent on the street level of Lincoln Way, also known as US Route 30, one of the main national highways crossing America from coast to coast. Our windows looked out on a slight slope in the highway where semi-truck diesel engines chugged their way uphill and “Jake-braked” downhill all day and all night, making sleep difficult. We got the famous book by Charles Goren and started playing Contract Bridge. I was never any good at it, but it helped to while away the evening hours. There was also a cute brunette from the neighborhood who used to drop by for a little flirting, but none of us ever dated her.
One of my roommates, a good-natured, blond, blue-eyed native of Minnesota showed us some photos of his family one night. There was an elderly man sitting in what looked like a tunnel near a furnace wearing an old-fashioned white sleeveless undershirt. “Who’s that old bastard?” I asked thoughtlessly. “Oh, that’s Dad,” my roommate said. There was an awkward pause in the room. I guess I was born with a gift for sticking my foot in my mouth.
We were all assigned to different groups in the Ames Lab. On my first tour of the lab I saw what had to be the world’s largest ion exchange columns. Now the rare earths were being separated in high purity by the kilogram. The columns were two or three stories high and about two or three feet in diameter. Just to fill such a column with the very pricey ion-exchange resin would probably have cost in the millions of dollars.

Ames Laboratory (Website)
My supervisor was a part-time Iowa farmer. We had several tea breaks every day, and got to know each other pretty well. Once he took me to his farm and I joined him and his wife for some great home cooking. He raised hogs and the best-tasting cantaloupes I ever had. The lab was full of oversized high-tech equipment, things like metal grinders. There were lots of gas cylinders, nitrogen, helium, argon. Money was clearly not a big issue. I had a chance to see many different rare earth metals on which we enjoyed the world monopoly. There were also some uranium ingots, mildly radioactive, sitting around like the two pound 3 or 4 inch disk-shaped paperweight I kept on my desk.
My main duty was taking large chunks of yttrium metal, breaking it into smaller chunks with a sledge hammer, and then putting the smaller chunks in a metal grinder under an Argon atmosphere. Like many metals yttrium is pyrophoric, and spontaneously catches fire when struck. I read in Wikipedia that my Caveman-like work was part of the lab’s production of 18,000 pounds of highly purified yttrium in the 1950s. Jim Miller recalls that he also worked on the yttrium project. What was it used for? Having a low neutron cross-section, I heard at one time that it was used for fuel rods of nuclear reactors. Since it is a decay product of uranium, yttrium is also produced by recycling spent fuel rods .
Today the AEC has been absorbed into the US Department of Energy. Ames Lab under a half-dozen new directors has continued to contribute to ever more sophisticated and difficult technological problems. My job was a worthwhile experience. I’m only sorry that the Ames Lab rare earth separation technology was allowed to slip away.
References:
1) Veronese, Keith. Rare: The High-Stakes Race to Satisfy our Need for the Scarcest Metals on Earth. Amherst, NY: Prometheus Books, 2015.
2) Johnston, David Cay. Free Lunch: How the Wealthiest Americans Enrich Themselves at Government Expense (and Stick You with the Bill). New York: Penguin Books, 2007, Chapter 4 on Chinese Magnetism.
3) Wikipedia – Ames Laboratory; Rare-Earth Element
